Quality And R & D
Ankang Zhengda Beijing Institute of Technology is the research and development institution set up by Ankang Zhengda Pharmaceutical in Beijing. As a domestic research window and high-end science and technology resource integration platform, it adopts an open and pragmatic cooperation mode, not for ownership, but for use. It integrates national medical research institutions and expert resources such as the Chinese Academy of Sciences, the Chinese Academy of Medical Sciences, the Chinese Academy of Military Medical Sciences, and establishes a domestic first-class research institute without walls to form a science and technology leading Empowering and innovative development, and taking the strategic varieties of Gynostemma pentaphyllum as the core, carry out in-depth research in the following fields.
(1) Carry out research on the planting resources of Gynostemma pentaphyllum, and research on the selection, optimization and planting technology of medicinal Gynostemma pentaphyllum varieties. (2) It is committed to improving the national quality standard of Gynostemma pentaphyllum saponins APIs and preparations, and the separation and purification of Gynostemma pentaphyllum saponin-A reference substance And structure confirmation. (3) Carry out systematic research and evaluation on chemical composition difference, material basis of efficacy, pharmacological mechanism, etc. of domestic Gynostemma pentaphyllum, and develop active drugs.The screening of the components lays a good foundation for the subsequent development of innovative drugs. (4) Carry out research on new dosage forms and expanded clinical indications of Gynostemma pentaphyllum saponins. (5) Carry out research on the comprehensive development and utilization of Gynostemma pentaphyllum resources. | 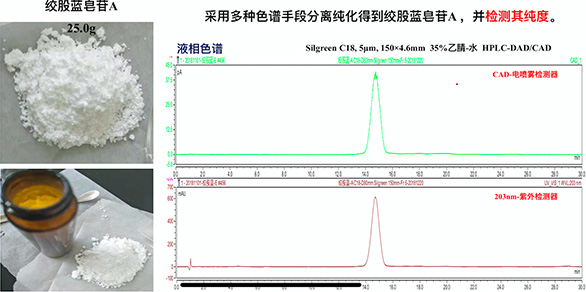 |
Gynostemma pentaphyllum saponin A reference substance - research achievements of Ankang Zhengda Pharmaceutical
In cooperation with the first-class Chinese medicine chemical laboratory in China, the reference gypenoside A was purified from the raw materials of Gypenosides and provided to the China Institute for Food and Drug Control for standardization and release, which solved the problem of preparation and supply of reference gypenoside A for the whole industry.

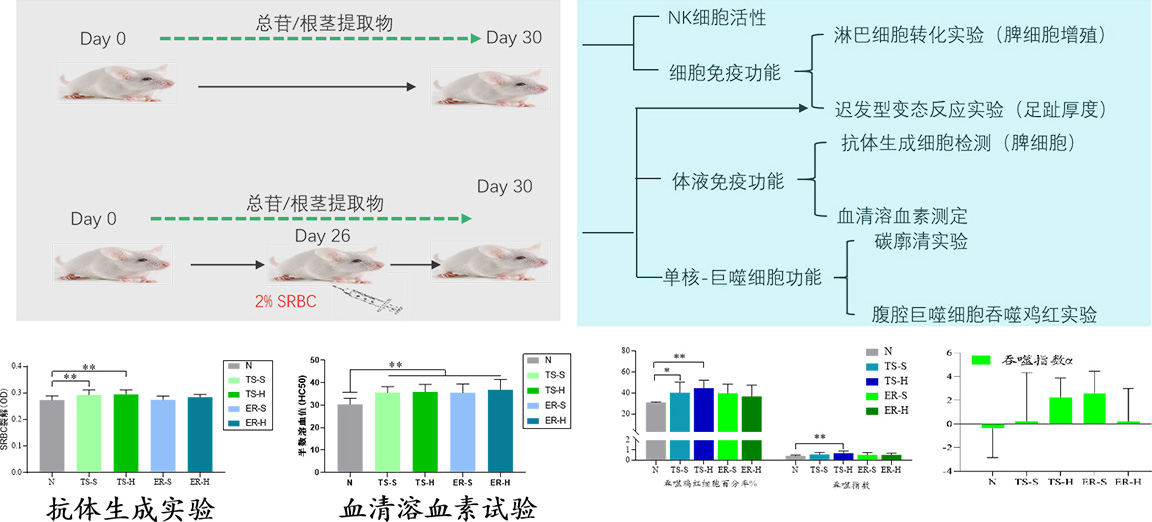
Gynostemma pentaphyllum extract enhanced immune test